Chemical engineers ‘borrow’ chemical engineering techniques to solve problem (Day 168)

11th November 2014

Collaboration and multidisciplinary study have been the buzzwords of research for a long time. But sometimes we forget how broad the field of chemical engineering is and that sometimes it is enough just to learn from other chemical engineers.
One of the common gripes I hear is that major companies are not willing to recruit chemical engineers from different sectors.
Perhaps this research from chemical engineers at Stanford University, who are applying petrochemical processing techniques to store solar energy, will make them think again!
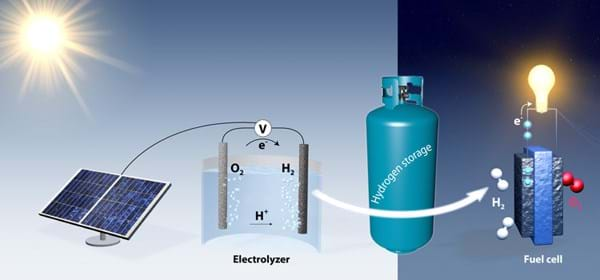
Chemical engineering Professor Thomas Jaramillo and his research associate Jakob Kibsgaard have created a catalyst that could help make large amounts of pure hydrogen through electrolysis (the method of running electricity through water to spilt hydrogen from oxygen found in water molecules).
Hydrogen is now considered a major commodity and normally comes from natural gas. Approximately ten million tons of hydrogen is produced a year in the US for use in petroleum refining and fertiliser production.
Thomas and Jakob aim to use electrolysis to produce hydrogen (H2) from water and use the process to help store solar energy, but made more efficient with a catalyst. However to use this process on an industrial scale, it must be cost-effective.
Platinum is considered to be the best catalyst for this process, but it is very expensive, thus the team have sought to find something else to use, Thomas said; “We're trying to make H2 in the most efficient way possible without using precious metals".
And Thomas and Jakob have succeeded, their work (published in Angewandte Chemie) details a version of molybdenum sulfide (they developed molybdenum phosphosulfide) – a cheap, durable and efficient catalyst that they think could replace platinum in this process.
Molybdenum sulfide is widely used in the petrochemical industries and has some similar properties to platinum but it is considerably cheaper.
Petrochemical processing has some similarities to electrolysis. Refineries use catalysts in reactions involving hydrogen to break down the feed stocks into lighter molecules like petrol. Electrolysis involves the breaking down of water molecules to create hydrogen.
The team’s work also uses another petroleum industry method, scrubbing the sulphur out of fuels to stop acid rain. In this petrochemical process the sulphur becomes incorporated in the catalysts, improving their efficiency.
So Thomas and Jakob tried adding sulphur to their catalyst and hence created molybdenum phosphosulfide, which speeds up the electrolysis reaction further.

Thomas noted these findings in this and earlier papers. But says that they are all based on petrochemical industry processes.
Thomas says that “It's exciting to make these connections between really different areas of technology,” he said, “and aim to operate at the meta-level of science.”
I think this is a lesson that we all need to learn.
*************************************************************************************************
Are you a chemical engineer who has switched your sector of work? Why not get in touch and tell us how you made applied your knowledge to new problems.